If you feel this doesn’t help you obtain a higher grade, simply email me within 7 days of purchase and I will grant a full refund.
Reaction Maps
Reaction maps for each functional group. Show more
- Summary of major reactions of each functional group that branches out in "web-like" fashion
- Covers all major functional groups in Org 1 and Org 2 (see "What's Inside" for more details)
- 15-page PDF (actually 60 pages compressed 50%) with full-color diagrams showing the reactions of each functional group, along with specific examples.
- Just a well-organized set of reactions of each functional group, as shown in "map"-like fashion
-
Immediate download for test prep -
Print ready PDF - nothing is mailed -
Contains key reactions covered in exams
Showing 4 of 12See All
Page 1 of 12

1. Index + Reactions of Alkanes
Index - Table of contents - Abbreviations for terms (e.g. Me, Et, Pr, Bu, Bn, Ph) and reagents (e.g. DCC, NBS, PCC, DIBAL, DMP, m-CPBA, etc) - Reactions of alkanes (halogenation) + specific examples
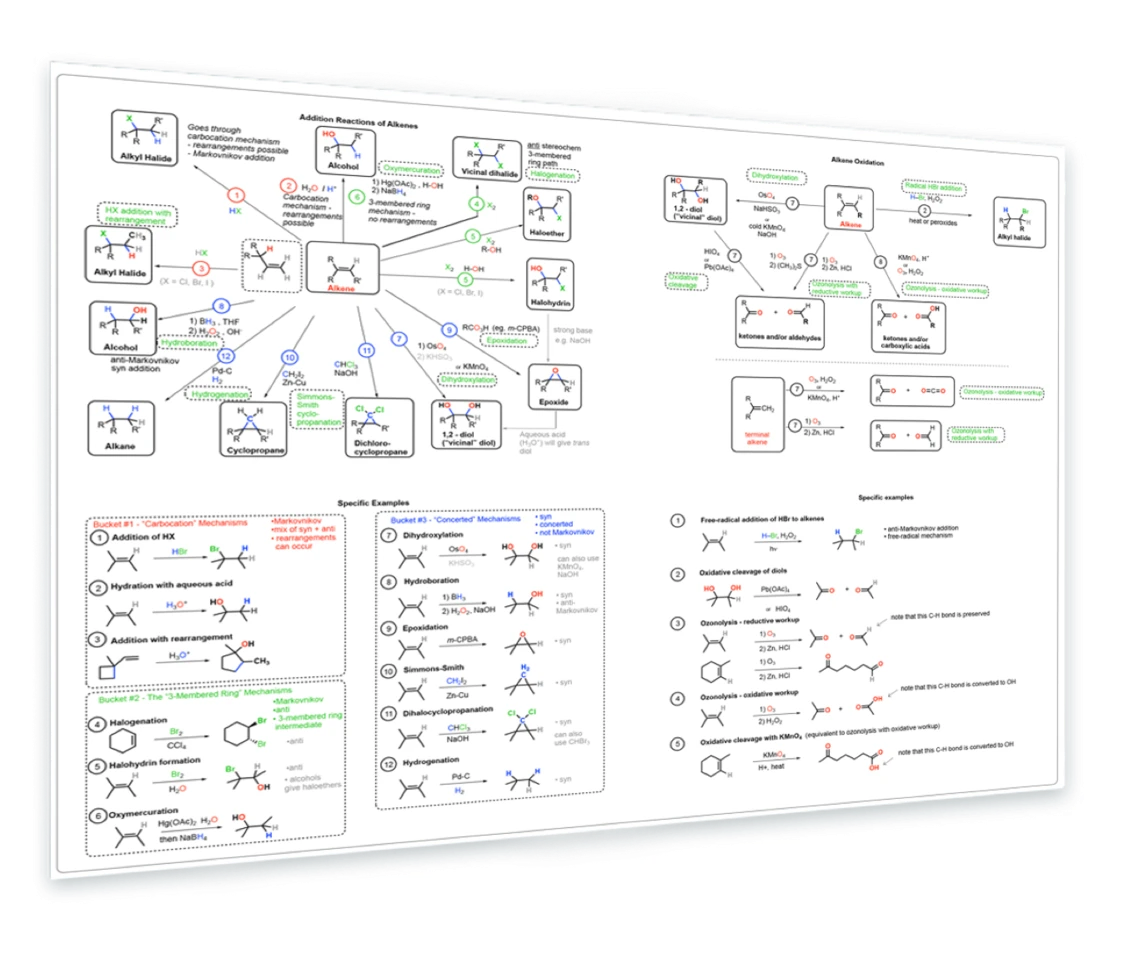
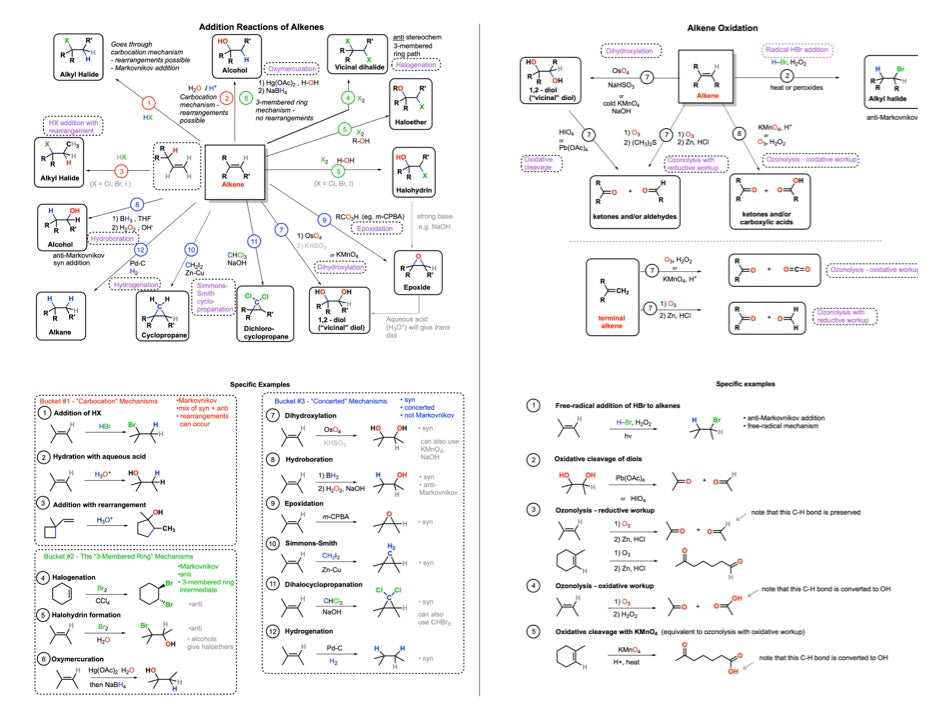
2. Reactions of Alkenes
Addition of HX - Addition of H3O+ - Addition of HX with rearrangements - Addition of Cl2, Br2 - Halohydrin formation - Epoxidation with mCPBA - Dihydroxylation with OsO4 - Hydroboration - Hydrogenation - Cyclopropanation - Ozonolysis - Specific examples
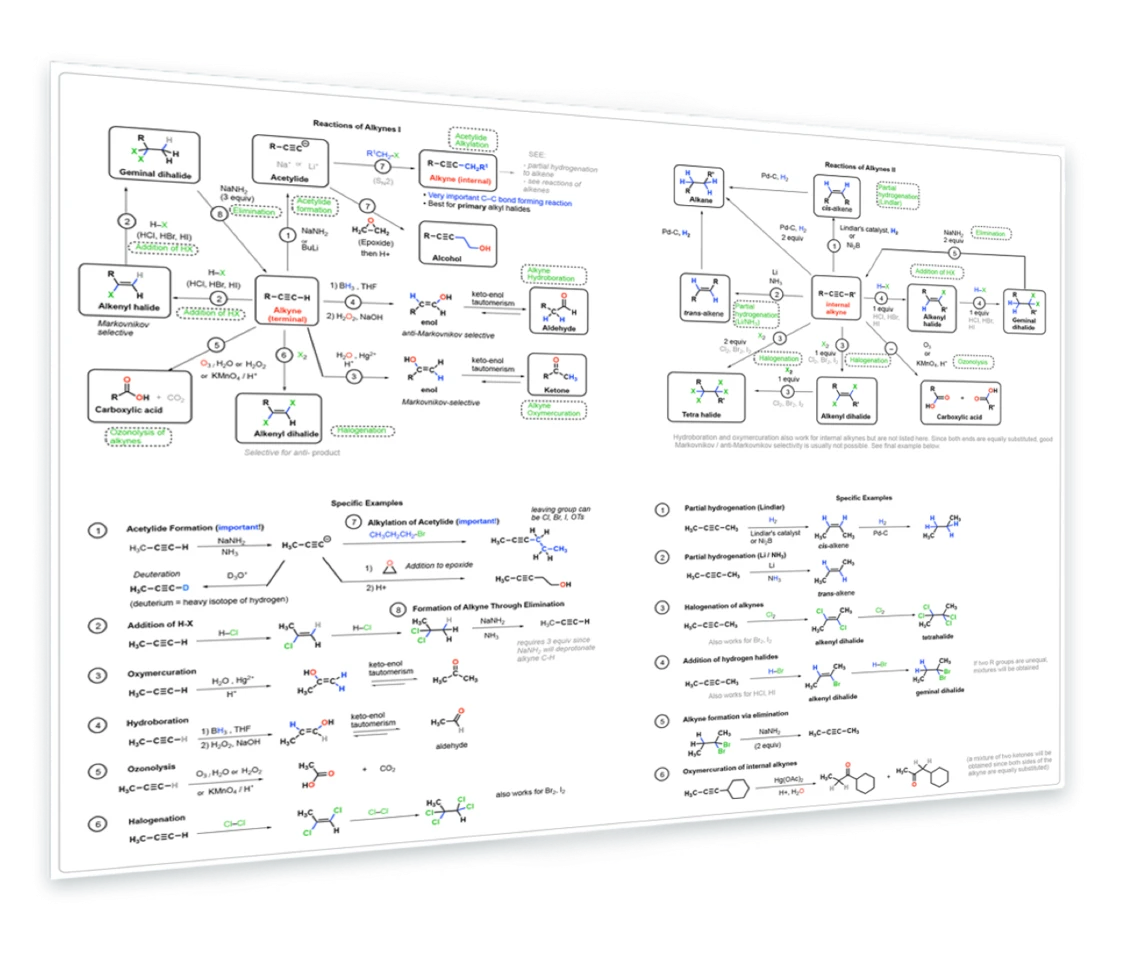
3. Reactions of Alkynes
Deprotonation - SN2 of acetylides - epoxide opening - hydroboration (R2BH) - oxymercuration - addition of HX - partial reduction (Lindlar + Na/NH3) - halogenation - ozonolysis with O3
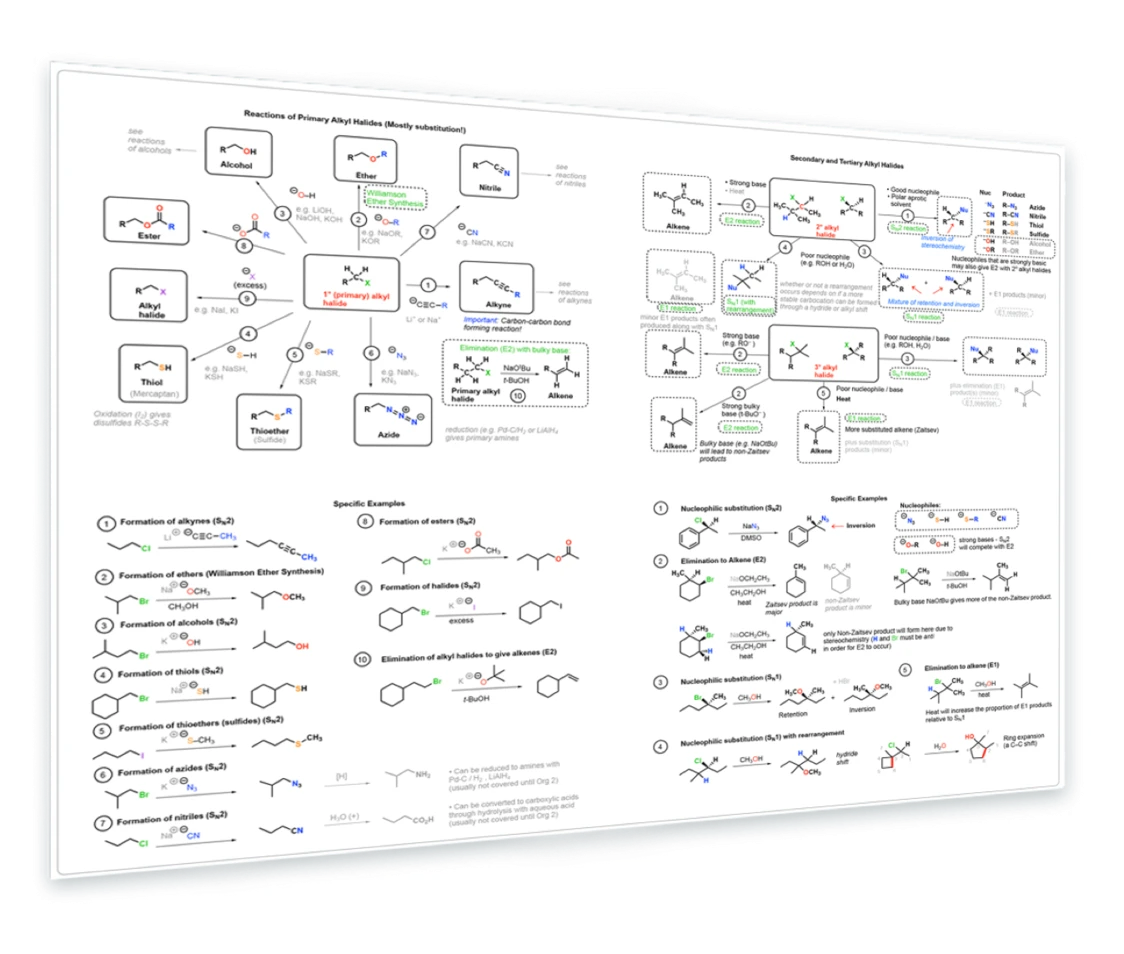
4. Reactions of Alkyl Halides
Primary alkyl halides - SN2 reactions with acetylides, RO- , HO-, HS-, N3, RS-, -CN - Secondary and tertiary alkyl halides - SN2 - E2 - SN1 with rearrangements - E1 - Specific examples included
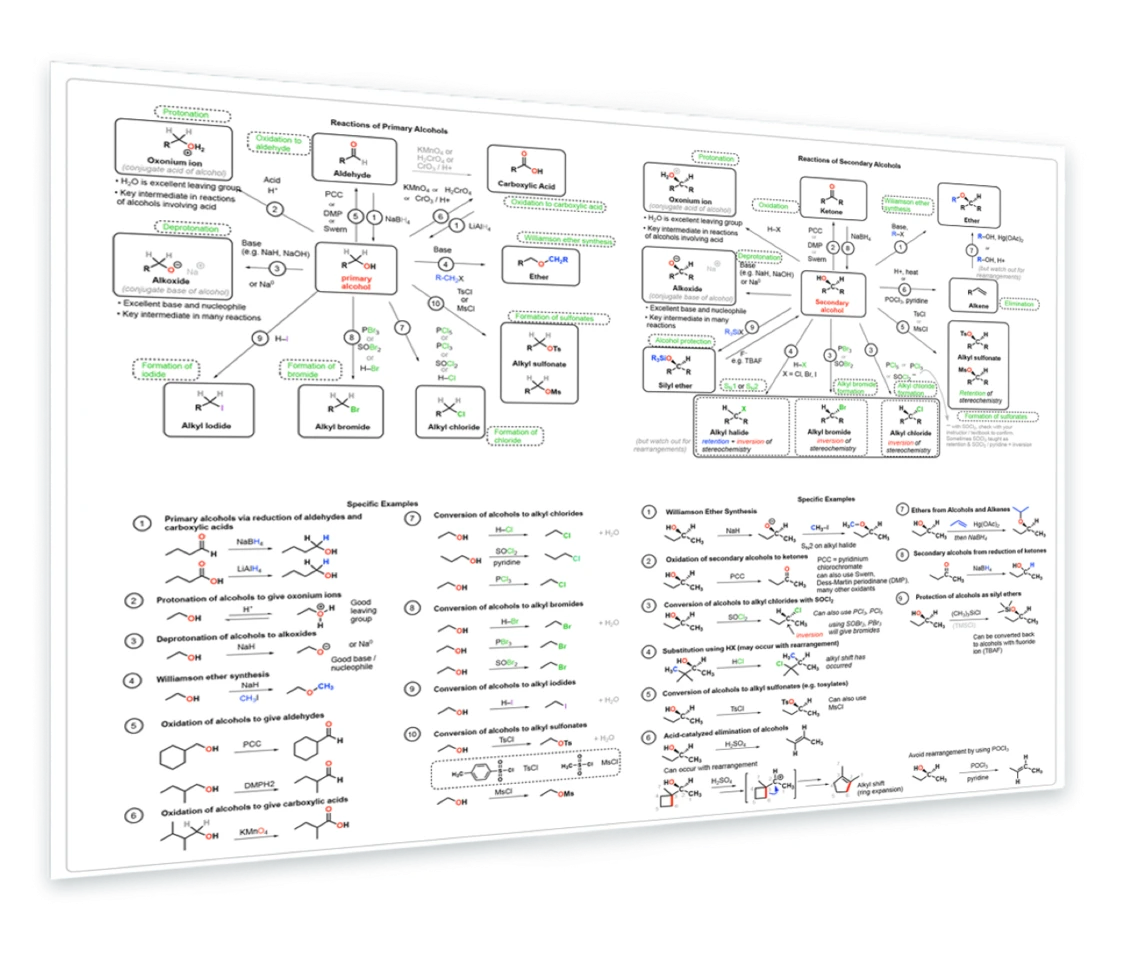
5. Reactions of Alcohols
Synthesis of primary alcohols - reactions of alcohols with acid and base - oxidation to give aldehydes and carboxylic acids - conversion to alkyl halides and alkyl sulfonates - reactions of secondary alcohols - Williamson ether synthesis - oxidation with PCC, DMP, Swern - conversion to alkyl halides - rearrangements
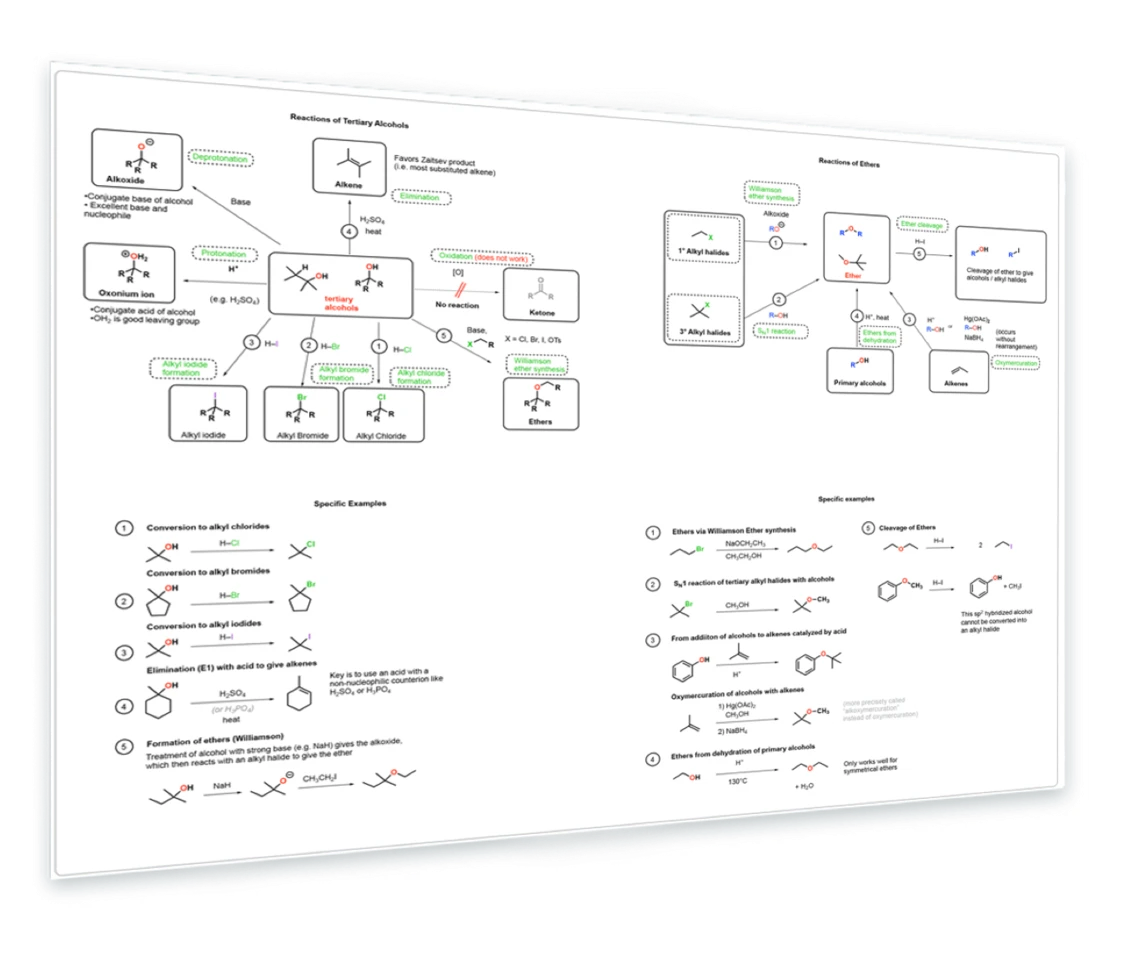
6. Reactions of Tertiary alcohols and ethers
Reactions of tertiary alcohols - conversion to alkyl halides - Elimination (E1) - Formation of ethers - Williamson ether synthesis - Ethers from SN1, addition to alkenes - Cleavage of ethers with acid
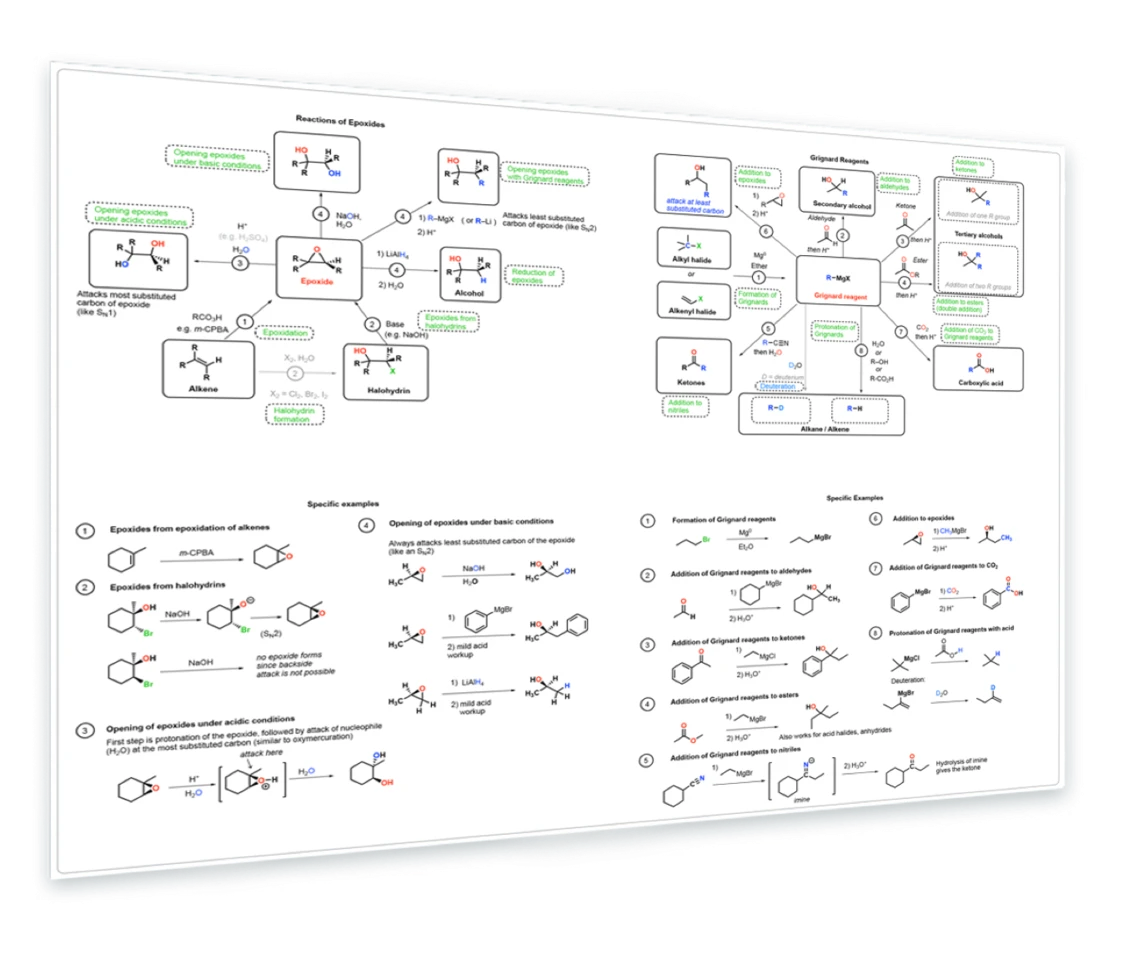
7. Reactions of Epoxides and Grignard Reagents
Synthesis of epoxides from alkenes - epoxides from halohydrins - opening of epoxides with basic nucleophiles (e.g. Grignards, NaOH) - opening of epoxides with acidic nucleophiles (H3O+) - Reactions of Grignard Reagents - Formation - addition to aldehydes, ketones, esters - much more
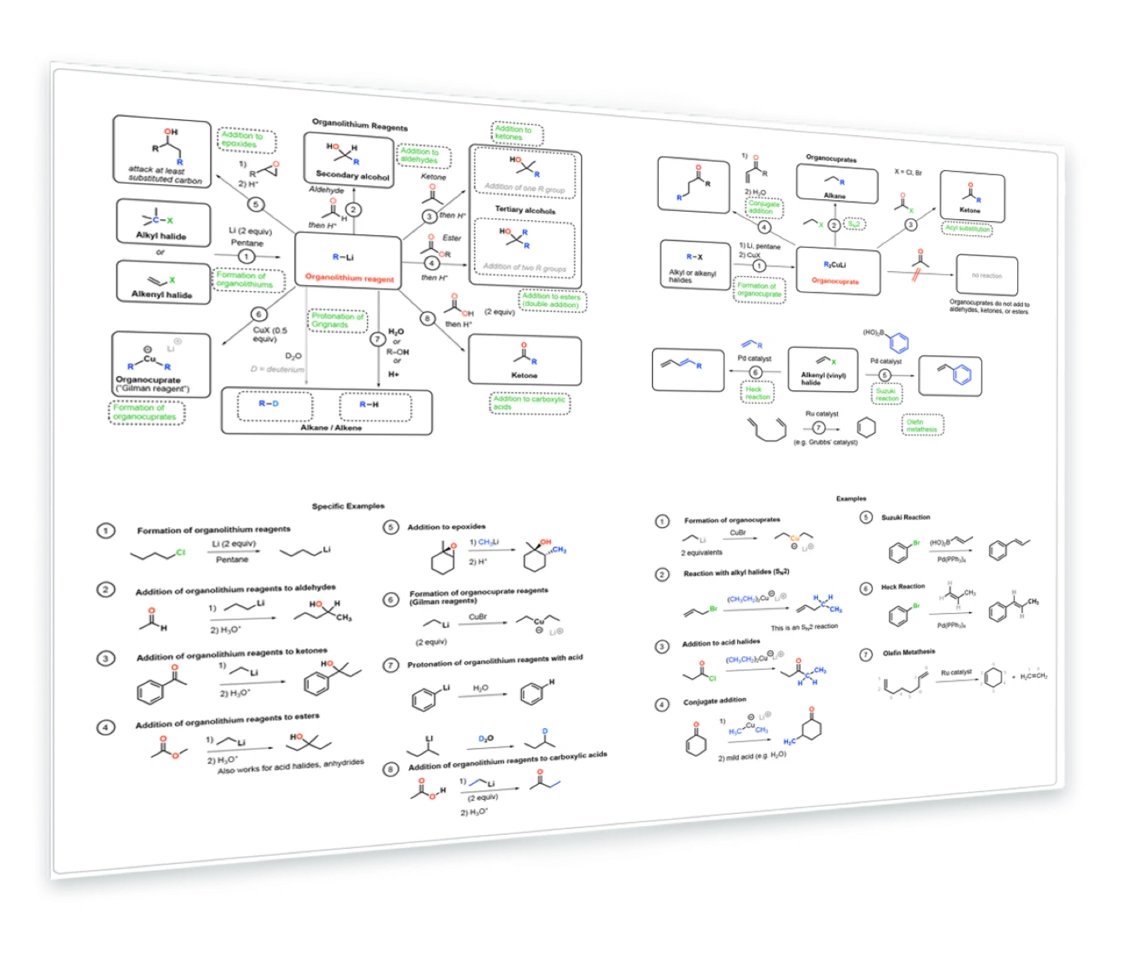
8. Reactions of Organolithium and Organocuprate Reagents
Formation of organolithium reagents - reaction of organolithium reagents with epoxides, aldehydes, ketones, esters, acids - Formation of organocuprates - Reaction of organocuprates with alkyl halides, acid halides, enones - Heck and Suzuki reactions - Olefin metathesis
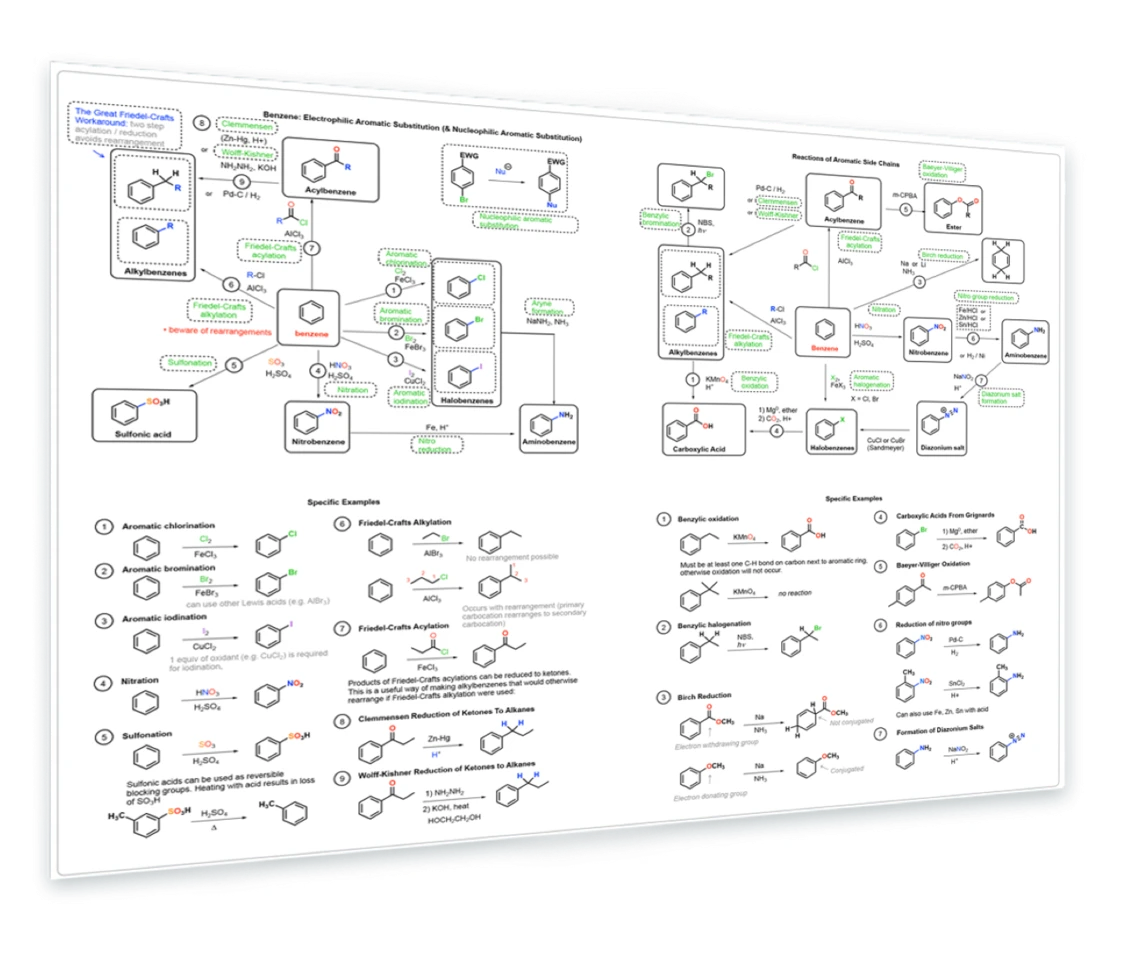
9. Reactions of Benzene and Derivatives
Electrophilic aromatic substitution - halogenation, nitration, sulfonation, Friedel-Crafts - Clemmensen reduction - Wolff-Kishner - Nucleophilic aromatic substitution - Birch reduction - Baeyer-Villiger oxidation - diazonium salt formation
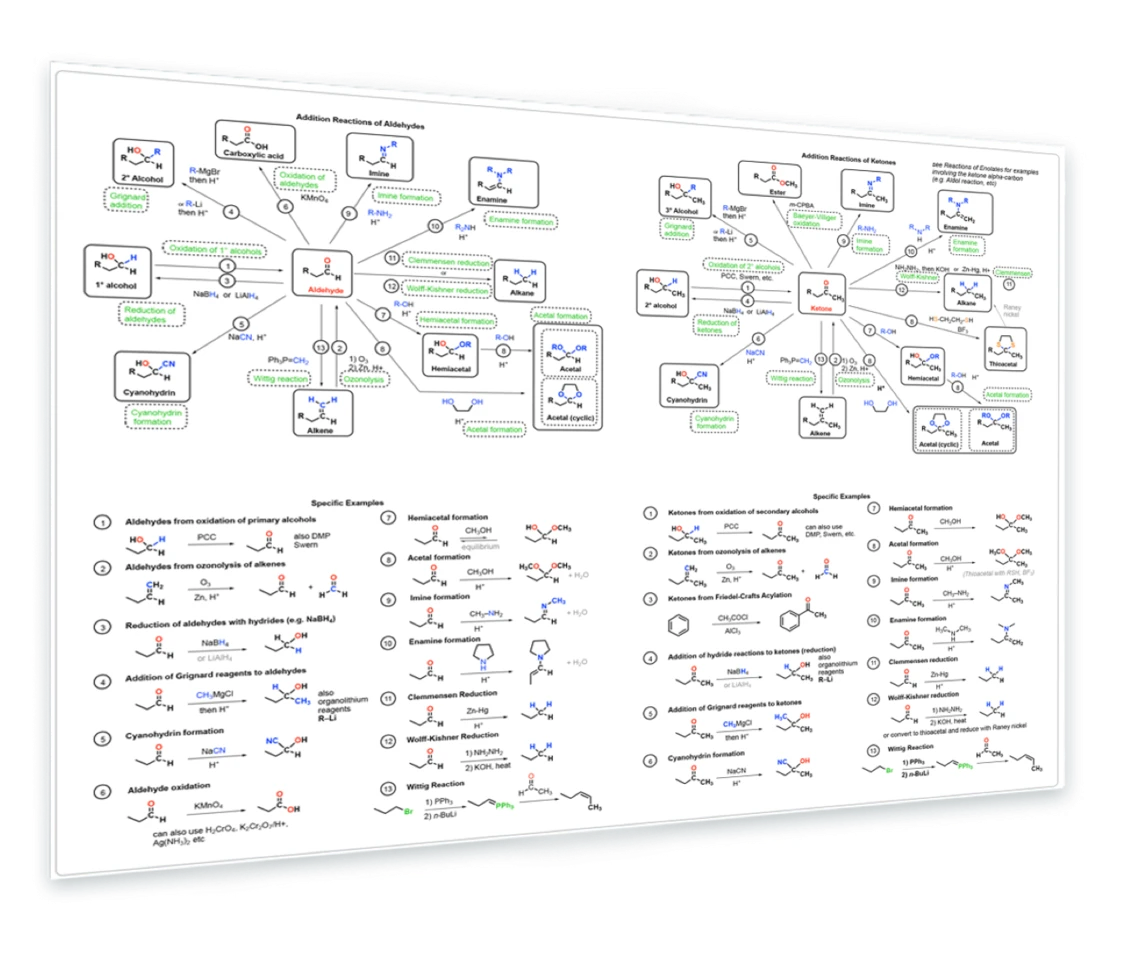
10. Reactions of Aldehydes and Ketones
Synthesis of aldehydes - Aldehydes from primary alcohols, alkenes - Reaction of aldehydes with Grignards - Reduction to alcohols - formation of hydrates, hemiacetals, hydrates, imines, enamines - Wittig reaction - reduction to alkanes
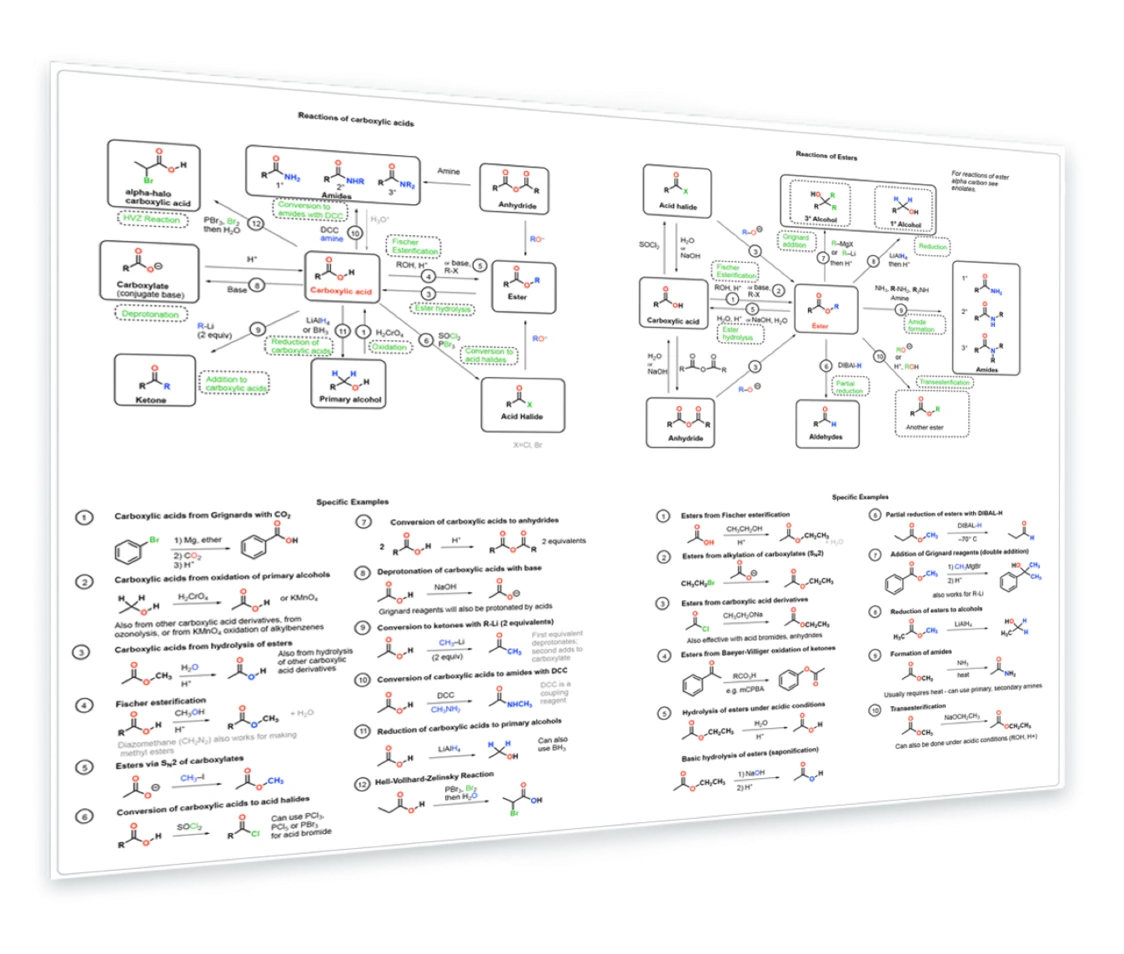
11. Reactions of Carboxylic Acids and Esters
Synthesis of carboxylic acids - Synthesis of esters - carboxylic acids via oxidation of primary alcohols - Fischer esterification - reduction of esters and carboxylic acids - reaction with Grignard and organometallic reagents
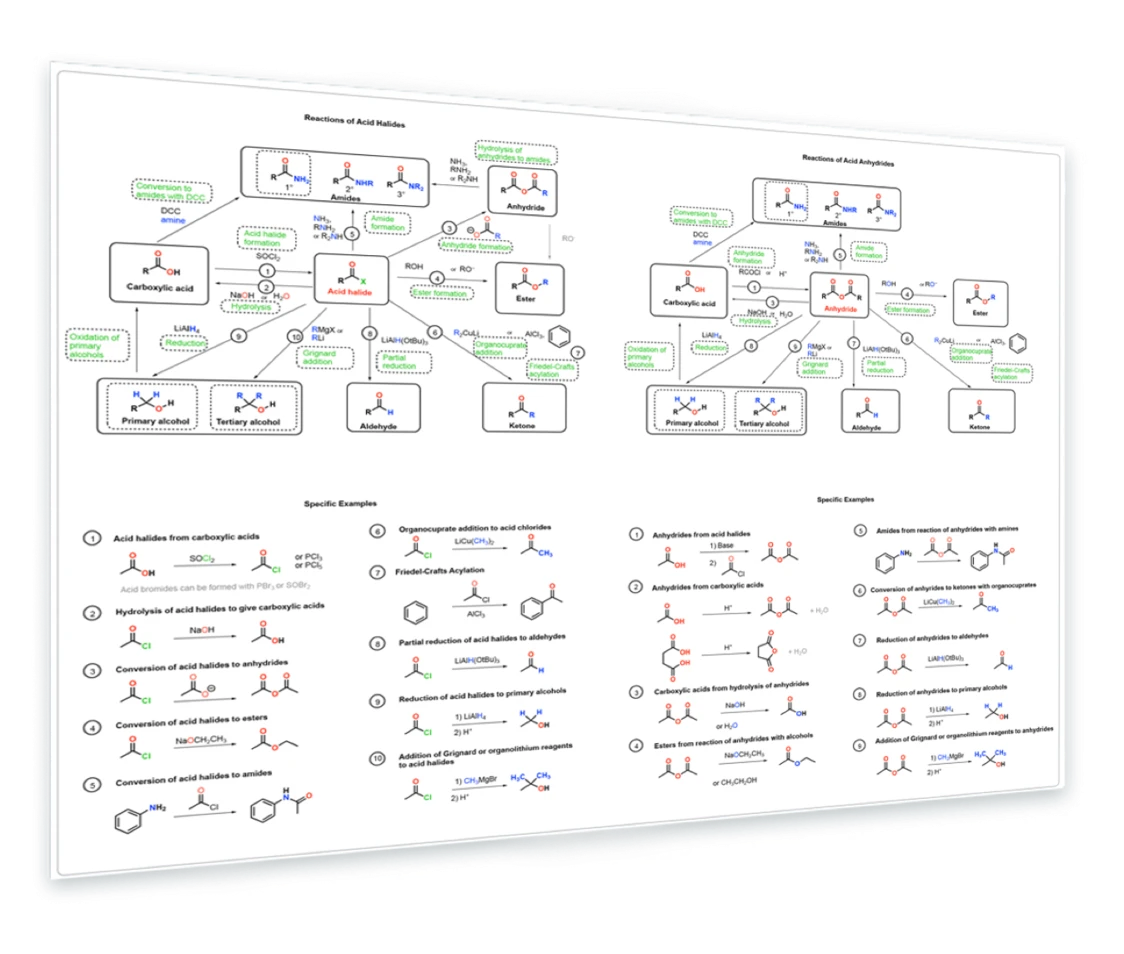
12. Reactions of Acid Halides and Anhydrides
Synthesis of acid halides - formation of esters, carboxylic acids, amides, and anhydrides from acid halides - Reduction of acid halides and anhydrides - Addition of Grignard reagents to acid halides and anhydrides - reduction
Your purchase is safe
- James Ashenhurst, Owner