If you feel this doesn’t help you obtain a higher grade, simply email me within 7 days of purchase and I will grant a full refund.
Org 2 Summary Sheets
A 20-page summary of key Org 2 Topics
Making your own notes is the best way to study organic chemistry. If you're finding that organic is coming at you pretty fast, however, this set of pre-made organic chemistry study notes (PDF) can help!
These notes clearly summarize each chapter down to a manageable number of concepts, give concise explanations to topics, are organized in a visually pleasing way that is easy to read and browse, and will help you see how the material in each chapter is connected. Also great for refreshing your o-chem knowledge!
-
Immediate download for test prep -
Print ready PDF - nothing is mailed -
Contains key concepts covered in exams
What's inside
Showing 4 of 12See All
Page 1 of 12

1. Introduction to Alcohols & Ethers
Alcohols and alkyl halides – Williamson ether synthesis – 3 more ways to make ethers - Epoxides from alkenes – Ring opening of epoxides under acidic and basic conditions – Oxidation of alcohols – Replacement of alcohols with halides using SOCl2 or PBr3 – Conversion to tosylates – Reduction of esters, carboxylic acids, aldehydes, and ketones.

2. Reactions of Dienes
Thermodynamic and kinetic control - Stability of alkenes and substitution - Isolated, conjugated, cumulated dienes - Resonance energy of dienes - 1,2 vs 1,4 addition to dienes - Kinetic and thermodynamic control in addition to dienes

3. Dienes: Molecular Orbitals & Conjugation
Conjugation and “Pi systems” - pi bonding - Conjugation of carbocations, radicals and lone pairs - Counting p-orbitals and pi electrons - Constructing pi molecular orbitals for the "allyl" system - Identifying the HOMO and LUMO for molecular orbitals
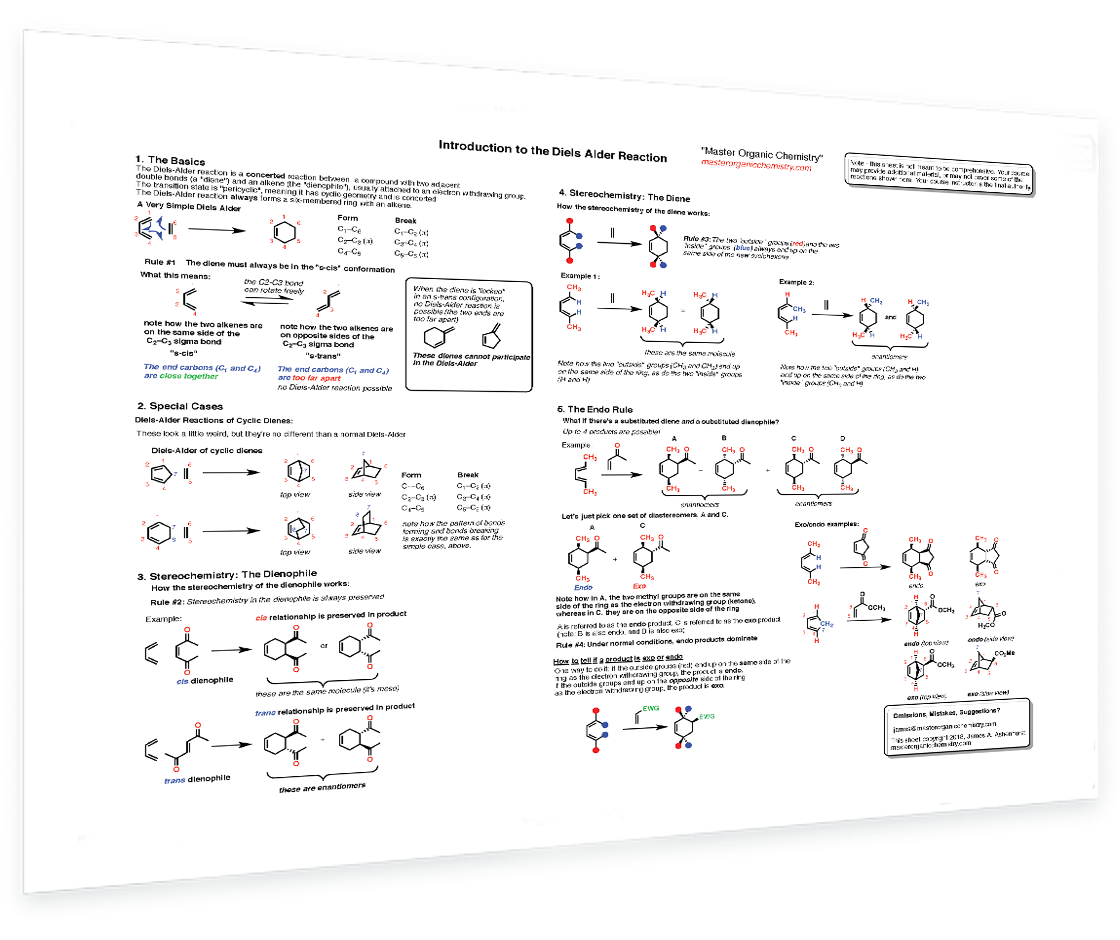
4. Diels Alder Reaction
Diels-Alder: Key bonds that form and break - Dienes and dienophiles - s-cis vs s-trans - Cyclic dienes - Stereochemistry of the dienophile - Stereochemistry of the diene - The endo rule - Identifyng exo and endo

5. Aromaticity
4 rules for how to tell if a molecule is aromatic, antiaromatic, or non aromatic – Resonance energy of benzene - Examples of aromatic compounds - Reactivity of aromatic compounds vs non-aromatic compounds - Addition vs substitution reactions - Antiaromaticity - Frost circles
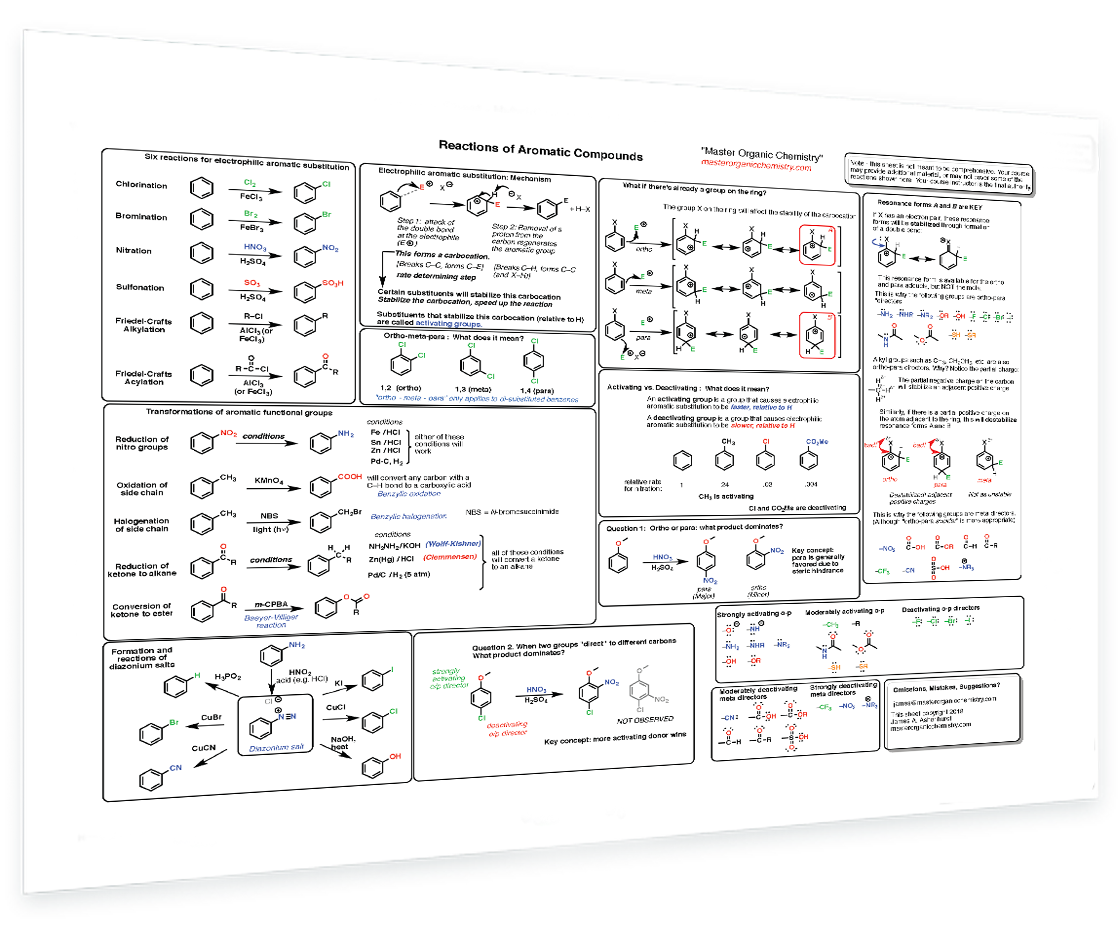
6. Reactions of Aromatic Compounds
6 key electrophilic aromatic substitution reactions - Mechanism of aromatic substitution - ortho, meta and para directing groups - Why are groups ortho para or meta directing? - Activating vs deactivating - Steric effects - Drawing key resonance forms - Reactions of side chains - What to do when there are 2 or more groups on the ring

7. Aldehydes & Ketones
A simple pattern for 7 key reactions of aldehydes - Electrophilic addition - Protonation - Grignard addition - 3 key factors affecting reactivity - Electronic and steric effects - Acid catalysis, synthesis by oxidation of alcohols - Synthesis from alkynes and alkenes - Protecting group formation - Imine formation

8. Key Reactions of Carboxylic Acid Derivatives
Nucleophilic acyl substitution - The importance of the leaving group base strength - The addition-elimination mechainsm - Carboxylic acids - Why do grignards add twice to esters - Double addition - Synthesis of carboxylic acids from alcohols - Deprotonation - Conversion to acyl chlorides -
Fischer esterification - Acidic hydrolysis of esters amides and nitriles

9. Enols & Enolates
Acidity of the alpha carbon - Structural features of the carbonyl group - Keto-enol tautomerism - Five factors that affect the keto-enol ratio – Factors that affect acidity of the alpha carbon - Acid catalyzes keto enol interconversion - Conjugate addition - Enolates as nucleophiles

10. Amines
Nomenclature of amines - How to understand amine basicity - Synthesis of amines from alkylation - Gabriel synthesis - Reduction of nitro groups - Reductive amination - Imine and enamine formation - Hofmann and Curtius rearrangements

11. Carbohydrates
What’s a carbohydrate? - The Fischer projection - D and L - aldoses and ketoses - Chain and ring forms of sugars - Reducing sugars - Haworth projections - Converting Fischers to ring forms - Reactions of carbohydrates

12. Carbonyl Reaction Mechanisms
9 key steps that make up all key reactions of carbonyl derivatives - Addition, elimination, protonation, deprotonation (proton transfer) – Keto-enol, 1,4 addition, 1-4 elimination, SN2
Your purchase is safe
- James Ashenhurst, Owner





